Abstract
Introduction
Hematopoietic stem cell transplant (HSCT) recipients experience periods of profound deficiency in both innate and adaptive immunity putting them at risk for a wide spectrum of infections, including organisms which are not normally pathogenic in immunocompetent hosts. Next generation sequencing (NGS) of plasma microbial cell-free DNA (mcfDNA) allows non-invasive untargeted diagnosis of human pathogens, making this modality appealing for this patient population (1). Here we perform a meta-analysis to evaluate the diagnostic value of NGS of plasma mcfDNA for infections in HSCT recipients.
Methods
We searched for relevant articles in BASE, PubMed, and ClinicalTrials.gov from inception to May 2022. Studies were eligible for inclusion if they assessed the diagnostic performance of NGS of plasma mcfDNA, included HSCT recipients with individual level data, and provided sufficient data to construct a two-by-two table, and were excluded if they were case reports or studies which only focused on the diagnosis of only one set of microorganisms. Two clinical adjudicators independently assigned the plasma mcfDNA test result as true positive, true negative, false positive, or false negative. The mcfDNA results were analyzed as a whole rather than by each individual organism if there was more than one organism reported. These data were then synthesized using random effect models and statistical analysis provided by the metadta module within the STATA statistical software package.
Results
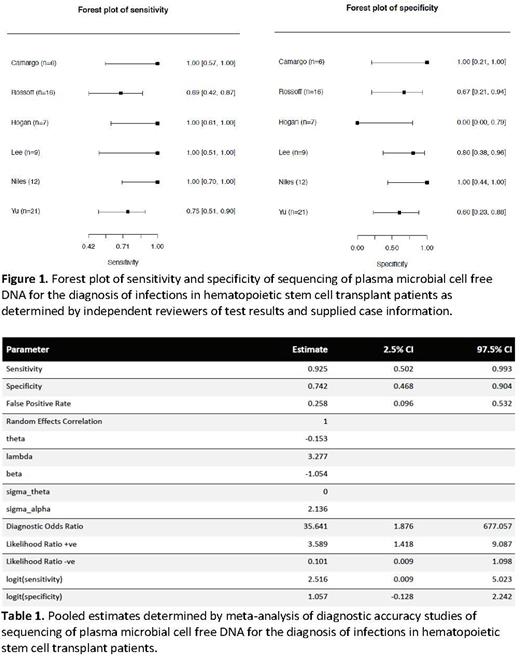
A total of 6 studies were included for the analysis, which included a total of 71 patients. The pooled sensitivity was 0.925 (97.5% CI 0.50-0.99) and the pooled specificity was 0.74 (0.47-0.90). The positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio were 3.59 (1.42-9.09), 0.10 (0.01-1.10), and 35.6 (1.9-677.1).
Discussion
NGS of plasma mcfDNA was effective in diagnosing infections in HSCT recipients. The high pooled diagnostic odds ratio found in this study suggests that NGS of plasma mcfDNA may have a unique role in this specific patient population, especially when the pre-test probability is high. The diagnostic accuracy of the test varies depending on host factors, preceding antimicrobial treatment, infection site, and the organism being evaluated. With a non-targeted test, it is possible that organisms are detected that may not be clinically significant, which would affect the test specificity, especially in populations with disrupted mucosal barriers, and therefore test results need to be carefully interpreted. The negative likelihood ratio of 0.10 suggests that there may be a role for negative tests as well, although this will need to be further explored. Overall, NGS plasma mcfDNA is a promising test for the diagnosis of infections in HSCT recipients.
References
1. Blauwkamp, Timothy A., et al. "Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease." Nature microbiology4 (2019): 663-674. 2. Camargo, Jose F., et al. "Next generation sequencing of microbial cell-free DNA for rapid noninvasive diagnosis of infectious diseases in immunocompromised hosts." Biology of Blood and Marrow Transplantation3 (2019): S356-S357.
3. Rossoff, Jenna, et al. "Noninvasive diagnosis of infection using plasma next-generation sequencing: a single-center experience." Open forum infectious diseases. Vol. 6. No. 8. US: Oxford University Press, 2019.
4. Hogan, Catherine A., et al. "Clinical impact of metagenomic next-generation sequencing of plasma cell-free DNA for the diagnosis of infectious diseases: a multicenter retrospective cohort study." Clinical Infectious Diseases2 (2021): 239-245.
5. Lee, Rose A., et al. "Assessment of the clinical utility of plasma metagenomic next-generation sequencing in a pediatric hospital population." Journal of clinical microbiology7 (2020): e00419-20.
6. Niles, Denver T., et al. "Plasma metagenomic next-generation sequencing assay for identifying pathogens: a retrospective review of test utilization in a large children's hospital." Journal of clinical microbiology11 (2020): e00794-20.
7. Yu, James, et al. "Impact of next-generation sequencing cell-free pathogen dna test on antimicrobial management in adults with hematological malignancies and transplant recipients with suspected infections." Transplantation and Cellular Therapy27.6 (2021): 500-e1.
Disclosures
Degner:Karius: Current Employment. Berman:Precision Health Solutions: Consultancy; Karius Inc: Current Employment. Simmons:Kite/Gilead: Speakers Bureau. Smollin:Karius Inc.: Current Employment. Morales:Karius: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal